Ochratoxin A is ubiquitous in cereals and products, milk, coffee, spices, alcohol and Chinese herbal medicines and their preparations, and can cause accumulation of toxins in humans and animals. Ochratoxin A has strong hepatotoxicity and nephrotoxicity and can cause teratogenic, mutagenic and carcinogenic effects in animals. Among the families of mycotoxins that have been discovered, OTA is considered to be the second only to aflatoxin, according to its importance and hazard. In view of the severe toxicity of ochratoxin A and the potential harm to humans, many countries and organizations around the world have given certain attention to it. According to the pollution situation of OTA and its toxicity, and referring to the foreign allowable limit of OTA, China has established the OTA limit standard for some foods. 4.5. Shanghai fly test biomycotoxin series fluorescence quantitative test strip product advantages Black Pepper,Black Pepper Powder,Chinese Black Pepper,Herbs Flavored Black Pepper Sichuan Liuhang Agriculture Co.Ltd , https://www.lhagriculture.com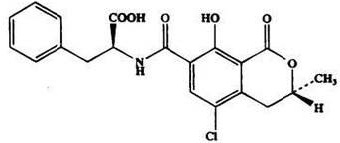


Contamination and Rapid Quantitative Detection of Ochratoxin A in Chinese Medicinal Materials - Accurate Quantification in -8min
I. Overview of ochratoxin
Ochratoxin mainly includes structural compounds similar to ochratoxin A, ochratoxin B, and ochratoxin C. Among them, OTA is the most widely distributed and most toxic, and it has the highest degree of pollution in crops. The study found that OTA has strong thermal stability and can only destroy a part after processing by baking, cooking and other processing, and the total content is only reduced by 20%.
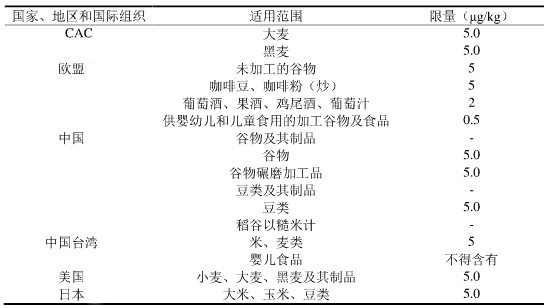
Second, some countries, regions and organizations limit the standard of ochratoxin A
Third, the study cases of ochratoxin by scholars from various countries
The earliest reports of ochratoxin A (OTA) contamination in medicinal plants were Croatian scholars. Of the 62 medicinal plants and 11 herbal tea samples they investigated, 11 medicinal plant samples contaminated aflatoxins. When these 11 positive samples were tested for aflatoxin, OTA and zearalenone, one of the samples was found to contaminate the OTA. Nigerian scholars investigated the production of mycotoxin in the storage period of medicinal plants and their products, and found that OTA increased with the increase of storage time, indicating that improper storage of medicinal materials is harmful to human health.
Chinese herbal medicines are prone to contaminate a variety of mycotoxins during planting, processing, transportation, storage, etc., seriously affecting their quality and efficacy. And different types. There are large differences in the residual levels of Chinese herbal medicines in different substrates and different medicinal parts, which increases the difficulty in detecting mycotoxins. Therefore, it is very important to establish a sensitive, accurate, rapid and efficient detection method for the level of mycotoxin contamination in Chinese herbal medicines and their products in combination with the corresponding detection techniques.
IV. Rapid quantitative detection scheme of ochratoxin A in Shanghai Feisheng Biological Chinese Medicinal Materials - Accurate quantification in -8min
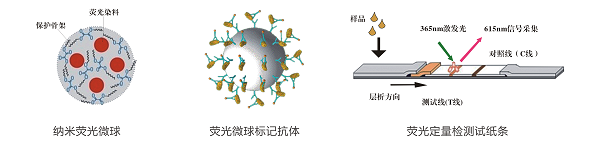
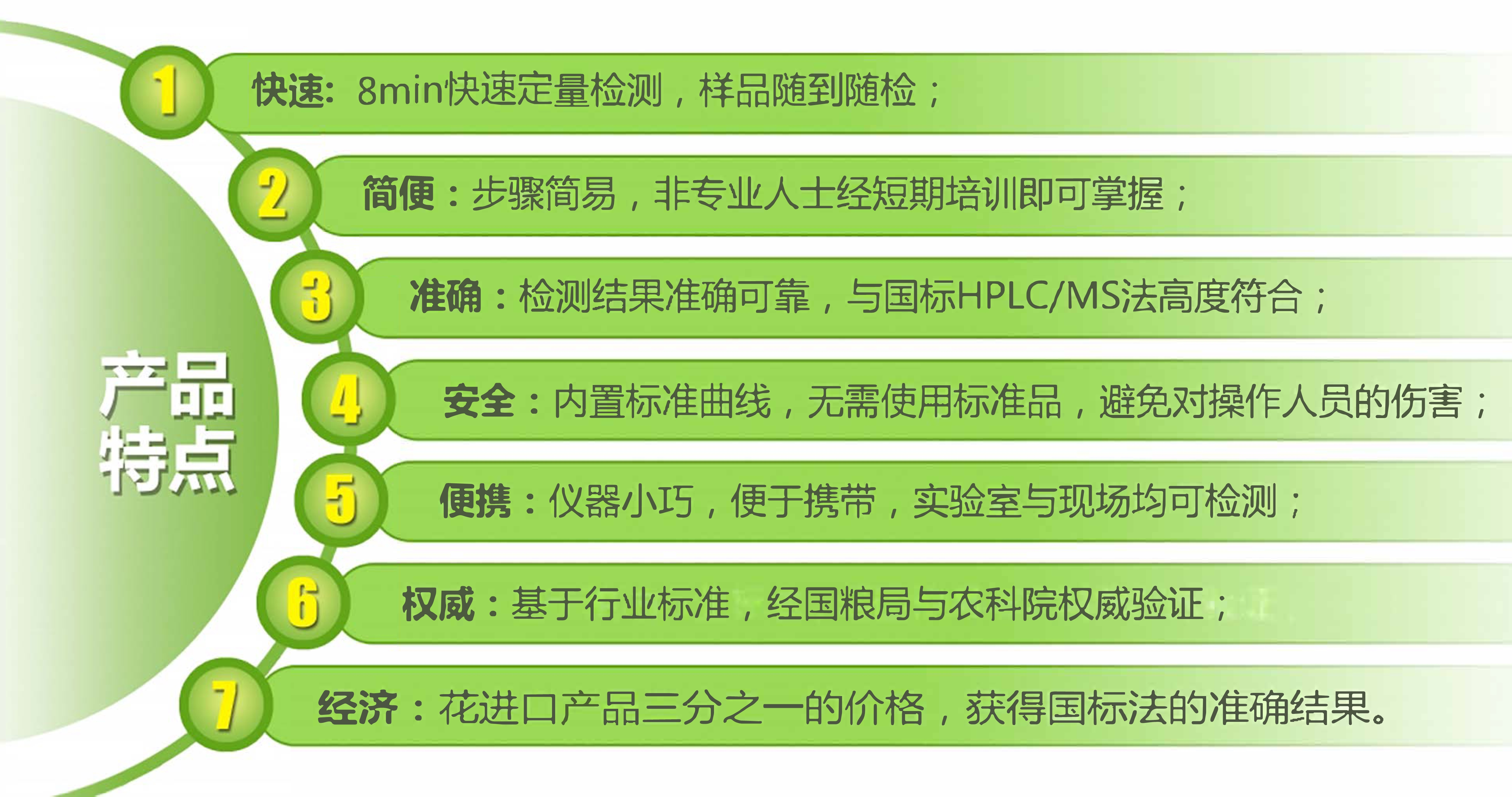
Based on the leading fluorescence quantitative FPOCT technology platform, Shanghai Festo Biotech is the first to introduce the ochratoxin A fluorescence quantitative rapid detection system, including ochratoxin A detector and ochratoxin A fluorescence quantitative detection strip, which can be fast in 8min. Accurate and quantitative detection of the residual content of ochratoxin A in Chinese herbal medicines, the sample preparation is simple, the detection operation is simple, the results are accurate and reliable, and can be printed on site. The accuracy is in accordance with the detection results of HPLC method, and is suitable for various Chinese herbal medicine processing enterprises. Third-party testing agencies and government regulatory agencies.
4.1. Ochratoxin A Fluorescence Quantitative Rapid Detection System Performance
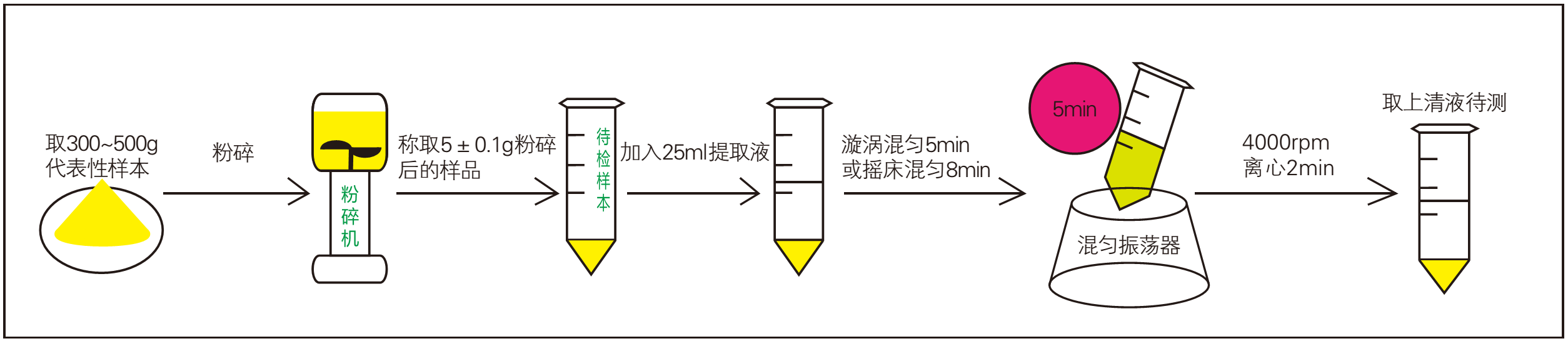
4.2. Sample preparation process
1. Crushing (pulverization and weighing of Chinese herbal medicine samples)
2. Oscillation extraction (5min);
3. Centrifugation (2min);
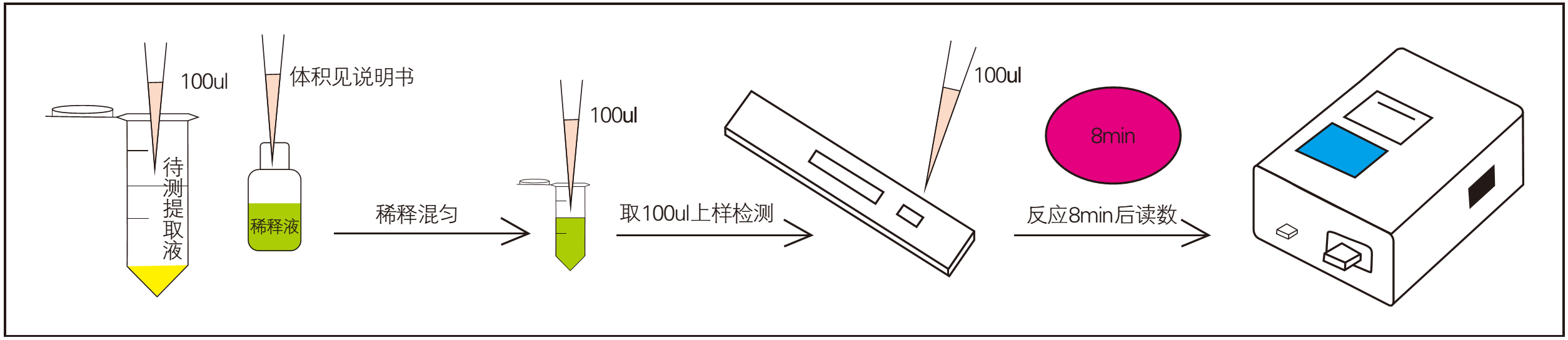
4.3. Detection operation process
1. Dilution;
2. Sample addition reaction (8 min);
3, reading, print test report;
4.4. Results interpretation and output
The portable ochratoxin A detector is used to make the readings more accurate and objective, and avoid human error.
The test result will be presented on the liquid crystal display of the fluorescence reader. At the same time, the paper test report can be obtained by pressing the print button. In addition, after the WIFI data upload function of the instrument is turned on, the relevant data information will be automatically uploaded to the “Food Safety Traceability Managementâ€. Cloud platform for easy traceability and quality management.