100mg
150mg
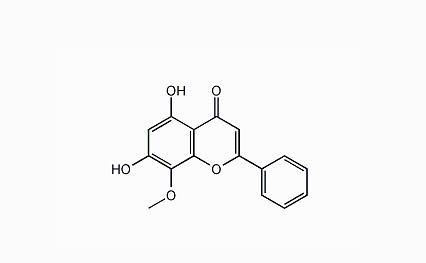
Neda Knibb (Nintedanib), a new generation of oral drugs for the treatment of carcinomatosis and idiopathic pulmonary fibrosis, is a three heavy vascular kinase inhibitor, which can simultaneously inhibit three major receptors, including vascular endothelial growth factor receptor (VEGFR), and platelet derived growth factor (PDGF), which lead to vascular regeneration and tumor growth. Body (PDGFR) and fibroblast growth factor receptor (FGFR).
In the treatment of idiopathic pulmonary fibrosis, Nidani can slow down the deterioration rate of idiopathic pulmonary fibrosis, which breaks through the traditional limitations of the medical profession.
The United States Food and Drug Administration (FDA) approved the treatment of idiopathic pulmonary fibrosis (idiopathic pulmonary fibrosis (IPF)) in October 2014. In Europe, Neda Knibb was also confirmed by the European Drug Administration (European Drug Administration) for special examination and approval. In June 2014, it was approved to be listed in the European Union region and was used as a Ofev product to treat idiopathic pulmonary fibrosis.

100mg
150mg
Neda Knibb (Nintedanib), a new generation of oral drugs for the treatment of carcinomatosis and idiopathic pulmonary fibrosis, is a three heavy vascular kinase inhibitor, which can simultaneously inhibit three major receptors, including vascular endothelial growth factor receptor (VEGFR), and platelet derived growth factor (PDGF), which lead to vascular regeneration and tumor growth. Body (PDGFR) and fibroblast growth factor receptor (FGFR).
In the treatment of idiopathic pulmonary fibrosis, Nidani can slow down the deterioration rate of idiopathic pulmonary fibrosis, which breaks through the traditional limitations of the medical profession.
The United States Food and Drug Administration (FDA) approved the treatment of idiopathic pulmonary fibrosis (idiopathic pulmonary fibrosis (IPF)) in October 2014. In Europe, Neda Knibb was also confirmed by the European Drug Administration (European Drug Administration) for special examination and approval. In June 2014, it was approved to be listed in the European Union region and was used as a Ofev product to treat idiopathic pulmonary fibrosis.
O-F-E-V Nintedanib The Treatment of Idiopathic Pulmonary Fibrosis
Model NO.: O-F-E-V
Delivery Time: 7-8 Days
Trademark: O-F-E-V
Transport Package: Discreet Package
Specification: 100mg 150mg
Origin: Germany
Model NO.: O-F-E-V
Delivery Time: 7-8 Days
Trademark: O-F-E-V
Transport Package: Discreet Package
Specification: 100mg 150mg
Origin: Germany