FDA approves cervical cancer vaccine Gardasil 9 to expand the applicable population: can be used for men and women aged 27-45
On October 5, the FDA approved a supplemental application for Merck's Gardasil 9 (9-valent recombinant human papillomavirus vaccine, HPV vaccine) for vaccination for women aged 27-45 and male adults. Prior to this, Gardasil 9 was intended for women aged 9-26 or male adolescents and adults, and the FDA did not approve any HPV vaccine for people over the age of 26.
Gardasil (4-valent HPV vaccine) was first approved by the FDA in June 2006. In December 2014, the FDA approved Merck's Gardasil 9 to prevent cervical cancer, vulvar cancer, vaginal cancer and anal cancer caused by HPV types 16, 18, 31, 33, 45, 52 and 58 and HPV6 and Type 11 caused by genital warts.
In addition to the four strains that Gardasil can immunize, Gardasil 9 adds five additional strains. Gardasil is also gradually discontinuing supply in the US market. In 2016, GSK's 2-valent HPV vaccine Cervarix (a bivalent human papillomavirus vaccination vaccine) also withdrew from the US market.
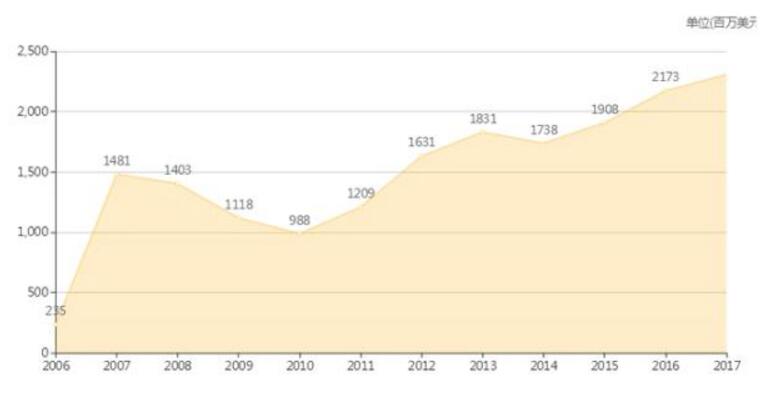
Merck's Gardasil/Gardasil 9 global sales over the years
Gardasil 9 covers the four strains of Gardasil and uses a similar manufacturing process as Gardasil, which the FDA considers to be comparable. In a large clinical trial of 3200 women aged 27-45 years with a mean follow-up of 3.5 years, Gardasil was used to prevent HPV infection, condyloma acuminata, vaginal precancerous lesions, cervical precancerous lesions, and cervical cancer associated with HPV infection. The effectiveness is 88%. The FDA's approval of Gardasil 9 for the 27-45 year old population is also based on the results of the above study and longer-term follow-up data for this study.
The safety of Gardasil 9 was evaluated in 13,000 women and men. Common adverse reactions included pain at the injection site, swelling and redness, and headache.
According to US CDC statistics, 14 million people in the United States are infected with HPV every year. 12,000 women are diagnosed with cervical cancer caused by certain types of HPV infection, and 4,000 women die from cervical cancer caused by certain types of HPV infection. In addition, HPV infection is also closely related to many other male and female cancers.
The above three vaccines have also been approved for marketing in China. Cervarix (bivalent human papillomavirus vaccination vaccine) and Gardasil (tetravalent human papillomavirus vaccine (Saccharomyces cerevisiae)) were successively approved by CFDA on 2016/7/12 and 2017/5/18, respectively. It took more than 10 years (see: 10-year market game for cervical cancer vaccine), and Gardasil 9 was approved on 2018/4/28 after special care. It only lasted 8 days from application to approval.
However, the three types of vaccines currently approved in China are not the same. Cervarix is ​​suitable for women aged 9-45, Gardasil for women aged 20-45 and Gardasil 9 for women aged 16-26. The HPV vaccine has not been approved for use in the male population in China.
Powder Trolley Fire Extinguisher
Powder Trolley Fire Extinguisher,Powder Fire Extinguisher External Type,Wheeled Dry Powder Fire Extinguisher,Dry Powder Trolley Fire Extinguisher
JIANGSU NEW FIRE FIGHTING TECHNOLOGY CO.,LTD , https://www.ffo2cylinder.com