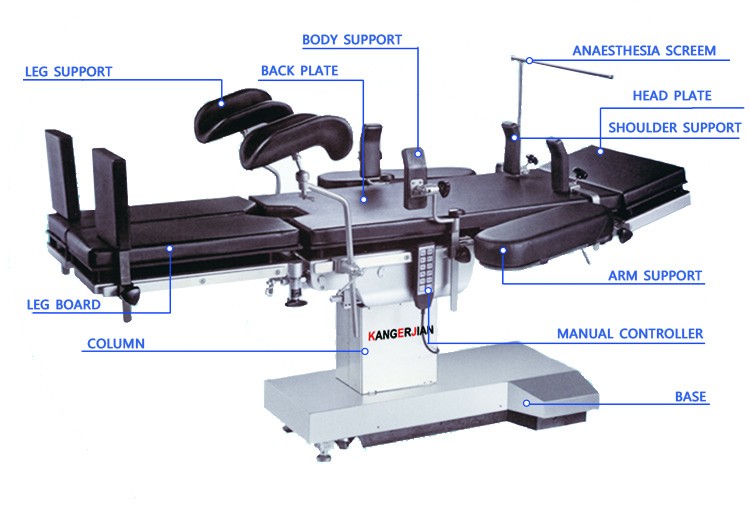
Electric Hydraulic Operating Table
1. Import hydraulic system
2. Memory sponge mattress
3. Electric Longitudinal displacement ≥350mm
4. Tabletop is X-ray available
5. Optional Carbon fiber bed panel
The Hydraulic system adopts imported integrated type components, imported motor and solenoid valve, stable performance.
The Operating Table uses wired mini touch controller.
Pillar base cover all 304 stainless steel, anticorrosion, easy to clean and durable.
Taiwan stainless steel structure, easy to use, safe and stable.
Accessories are removable.
The Multi function medical operating table is suitable for comprehensive surgical operations like thoracic surgery, abdominal surgery, brain
surgery, eye surgery, ENT, gynaecology and obstetrics, urology surgery, orthopedics surgery and other general purpose.
More Images:
The brief medical operating table without accessories installed on
The whole set Surgical Operation Table with all related Accessories intalled on
Certificates:
Certificates of CE, ISO9001, ISO13485 and CFDA are approved
Company:
Shangdong province is the main machinery production base in China.
KANGERJIAN Medical Technology Co., Ltd. is a group of senior lighting design expert and machinery manufacturing expert company with 20years experience and factory locating in the east city--the hometown of confucius--Qufu in Shandong province, China.
The Company has passed the ISO:9001:2008 quality system certification, ISO13485:2003 quality system certification, CE certification and CFDA certification, so that the enterprise management standards and product quality is relatively connected to expand the international market for enterprises to lay the foundation.
Our main products: Operation Theatre Lights , Operating Room Lights , Double Dome Halogen Operating Light, Single Dome Halogen Operating Light, LED Operating Light , Surgical Operating LED Light , Mobile Type Operating Light , Gynecology Examination Tables , Obstetric Delivery Bed , Electrical Gynecological Table , Obstetric Delivery Table , Delivery Examination Table , Electric Hydraulic Operating Table, Electric Medical Operating Table, Manual Electric Operating Table , Surgical Table , Operating Table, Operating Theatre Pendants , icu tower crane in ICU room, LED Viewbox etc. professional medical equipment.
The quality of casting by me, the market led by me! Excellent from professional, KANGERJIAN people lead the new trend of medical equipment.
Electric Hydraulic Operating Table Electric Hydraulic Operating Table,Electric Hydraulic Operating Bed,Hospital Electric Hydraulic Medical Table,Hydraulic Pressure Operation Bed Shandong Kang'erjian Medical Technology Ltd. , https://www.operatingtable.nl
Build "group advantage"
At the opening ceremony of the Fourth China-US Pharmacopoeia International Forum, Roger L, MD, CEO of the US Pharmacopoeia Commission. Williams said that since patients and consumers around the world rely on the world's drug and food standards agencies to establish strong standards, the cooperation between these Pharmacopoeia development agencies must be closer than ever before to solve related technical and resource challenges.
Williams said: "As the countries around the world are trying to set and implement standards that allow drug regulators, manufacturers, and patients and consumers to believe that they can ensure the quality and safety of pharmaceuticals, pharmacopoeia institutes are faced with an increasing number of technologies In this context, pharmacopoeia-regulatory agencies around the world only have to work more closely together to generate group advantages. If we want to meet the urgent health needs of these global public in a timely manner, we must cooperate more closely."
Williams promises that in the future, the United States Pharmacopoeia Commission will increasingly actively cooperate with national Pharmacopoeia development agencies in the world. In his speech, he also recalled and spoke highly of the cooperation between the United States Pharmacopoeia Commission and the China Pharmacopoeia Commission. These cooperations mainly include five aspects, namely, the China National Pharmacopoeia Committee and the China National Institute of Food and Drug Control (formerly the China National Pharmaceutical and Biological Products Examination. Institute of Nutrition and Food Safety, China CDC, and the Institute for Drug Control in Shanghai, Zhejiang, Beijing, and Guangzhou signed a memorandum of understanding; employed eight prominent Chinese experts to become members of the Standardization Committee of the US Pharmacopoeia Commission; A large number of Chinese technicians participated in the Visiting Scientist program of the United States Pharmacopoeia Commission; the standards of the United States Pharmacopoeia Commission were translated into the official Chinese language; laboratories worked together to make reference standards for the US Pharmacopoeia Commission, and were used by global manufacturers and regulators. Used to help ensure the quality of medicines and foods. These cooperation have played an active role in enhancing mutual understanding and promoting the improvement of quality standards.
Internationalization of Chinese medicine standards
Chinese medicine is a cultural treasure of the Chinese nation and has made enormous contributions to the prosperity and prosperity of the Chinese nation for thousands of years. The Chinese medicine industry is one of the few industries in China with international comparative advantages. To let the Chinese medicine industry go international is a great cause that benefits the nation, benefits the country, and benefits humanity. However, there is a big difference between traditional Chinese medicine and Western modern medical systems. It lacks the support of systematic modern scientific clinical research data, and at the same time it is difficult to make scientific representations using modern medical theories. These factors directly hinder Chinese medicine products from entering the international market. . What's more, traditional Chinese medicines do not yet have an internationally recognized uniform quality standard, which directly leads to difficulties for Chinese medicine to go abroad.
The 2010 version of the Chinese Pharmacopoeia that has just been formally implemented contains a significant increase in standards for extracts of Chinese herbal medicines, Chinese herbal medicines, Chinese patent medicines, and extracts of traditional Chinese medicines. This has changed and reversed the long history of the Chinese Pharmacopoeia. The lack of a standard situation for Chinese Herbal Pieces has laid a good foundation for the internationalization of Chinese medicine. For example, the new version of the pharmacopoeia significantly increased the specific identification of Chinese medicines. Color and precipitation chemical reactions and spectral identification methods are no longer used in Chinese medicine standards. The standard greatly increased cross-section or powder microscopic identification. In the 2005 edition of the Pharmacopoeia, 620 microscopic identifications were included; in the 2010 edition of the Pharmacopoeia, only 636 microscopic identifications were added. All medicinal materials and decoction pieces and proprietary Chinese medicines containing raw powdered medicines have basically increased specific cross-sections or Powder microscopic identification. At the same time, the TLC identification technology is highly used in the new standard. In the 2005 edition of the Pharmacopoeia, a total of 1,507 items were identified by thin-layer chromatography; the 2010 edition of the Pharmacopoeia only identified 2,494 items by thin-layer chromatography. In addition to mineral medicines, there are specialized thin layer identification methods.
In fact, one of the topics of the International Forum on Chinese and American Pharmacopoeia is the standardization and quality control of traditional Chinese medicine. Chinese medicine wants to go out of the country and onto the world stage. The internationalization of quality standards is the threshold that must be overcome. Applying for FDA certification in the United States is one of the main channels for the internationalization of Chinese medicines. If Chinese medicine preparations pass the FDA's listing approval, it means that they have obtained access permits for entry into the international mainstream pharmaceutical market.
At the forum, Qian Zhongzhi, Director of the Chinese Medicines Standards Division of the National Pharmacopoeia Commission, said that the Chinese Pharmacopoeia Commission has recently provided the United States Pharmacopoeia Commission with standards for 99 varieties of Chinese medicines and 11 varieties of chemical drugs. The US Pharmacopoeia Committee is reviewing and amending It was determined that the five Chinese medicinal plants, Andrographis paniculata, Centella asiatica, Cinnamon, Artemisia annua, and Ganoderma lucidum, were included in the US Pharmacopoeia Dietary Supplement Code. In addition, the European Pharmacopoeia (the official Pharmacopoeia that is enforced by law in EU member states) also contains four varieties of Chinese medicinal herbs, which are Panax pseudoginseng, ginseng, safflower, and mantle. This means that Chinese medicine has become international as a traditional Chinese medicine.
At the forum, experts from the United States Pharmacopoeia Commission Dietary Supplements Expert Committee also introduced the Chinese-U.S. Pharmacopoeia's cooperation in Chinese medicine. At present, the Chinese Pharmacopoeia Commission has recommended that the United States Pharmacopoeia earn 99 varieties of Chinese medicine. In the next step, the Chinese Pharmacopoeia Commission will provide drafts, notes and samples of relevant standards. The US Pharmacopoeia Commission will then validate the methods and data. In the future, the China-US Pharmacopoeia Commission will discuss relevant work in a timely manner by holding annual meetings and publish the results of the work for public review.
At the forum, Dr. Guo Dean of the Chinese Medicine Modernization Research Center of the Shanghai Institute of Pharmaceutical Sciences of the Chinese Academy of Sciences pointed out that the main challenges facing modernization of traditional Chinese medicine include maintaining the stability of Chinese medicine quality, clarifying active ingredients, evaluating overall safety, providing clinically valid scientific data, and constructing Suitable animal models, development of modern Chinese medicine products, etc. The premise of the internationalization of traditional Chinese medicine is to provide scientific basis for traditional Chinese medicine with safety, effectiveness, and quality controllability, so that traditional Chinese medicine can also become evidence-based medicine.
Biopharmaceutical opportunities currently
Another topic of the International Forum on Chinese and American Pharmacopoeia is the standard and production quality control of biological products. Up to now, the global biopharmaceutical industry has expanded at a rate of more than 15% for more than 10 years in a row, becoming one of the fastest growing high-tech industries. In 2007, biopharmaceutical global sales reached 84 billion U.S. dollars. Despite the "global financial crisis", the global biopharmaceutical market still exceeded $90 billion in 2008.
The rapid growth of the biopharmaceutical market is an important development opportunity for local companies. The "Policy for Accelerating the Development of the Biological Industry" promulgated in June 2009 clearly stated that it will focus on the development of new vaccines and diagnostic reagents for preventing and diagnosing serious infectious diseases that seriously threaten the lives and health of the people of our country; active research and development will treat common diseases. Major diseases have significant curative effects on biotechnology drugs, small molecule drugs and modern Chinese medicine; accelerate the development of biomedical materials, tissue engineering and artificial organs, clinical diagnosis, treatment and rehabilitation equipment; and promote biomedical R&D outsourcing.
In this context, strict quality standards and production control of biological products have become the basis for the development of China's biological products industry. At the forum, Li Fengxiang, director of the Department of Health Inspection of the China Food and Drug Testing Institute, said that strict quality standards and production control of biological products are very important because certain biological products, especially preventive products, are used by healthy people and infants. Young children. The production control of biological products has its own characteristics, for example, the production process is a biological process, it is a sterile operation process, and attention needs to be paid to biological activity control and the like. Therefore, for the quality control of biological products, attention should be paid to the consistency of the production process and approval, the correctness of the use of raw materials, the ratio of feed and output, the analysis of key quality control items and trends, and the homogeneity of products.
Yang Xiaoming, general manager of China Biotechnology Group, introduced the quality control of vaccine production in China. He said that the current level of Chinese vaccine quality has been in line with international standards. The current production facilities of vaccine manufacturers have all implemented cGMP standards. Some companies have already started pre-certification of WHO, and the JEHA live vaccine produced by Chengdu Institute of Biological Products is expected to become China's The first vaccine product to be pre-certified by WHO.
Scientists have long predicted that the 21st century is the century of information technology and biotechnology. Biotechnology has changed human life in many ways and is particularly prominent in medicine. There is even a saying that if there is no biotechnology, the pharmaceutical industry is ultimately a boring career. People have reason to believe that in line with international standards in the quality standards and production quality control, will greatly help local companies to seize the rare opportunities for the development of international biopharmaceuticals.







Complementary Chinese medicine internationalization standards bring Chinese pharmaceutical opportunities
According to the relevant spirit of the memorandum of cooperation between the Chinese and US Pharmacopoeia Commission, a few days ago, the China Pharmacopoeia Commission (ChP), together with the United States Pharmacopoeia Commission (USP) and Zhejiang Food and Drug Administration, hosted the Fourth China-US Pharmacopoeia International Forum in Hangzhou. Wu Hao, the deputy director of the State Food and Drug Administration attended the forum and delivered a speech. Wu Hao pointed out that the development of the world's pharmaceutical industry needs to strengthen international exchanges and cooperation in drug standards. The issue of drug quality and safety has become a global issue of common concern, and countries need to strengthen cooperation and respond together. Each country's pharmaceutical industry has its own characteristics, and it should take the road of "complementary advantages and common development."